We all love sugar, especially when it's in the form of a caramel or a butterscotch-type "quick caramel" sauce. Caramel drizzled over cakes is the best, and ice cream too, or baked apples. You can even layer caramel into cakes. Unfortunately, sugar can be a real pain to work with.

In the kitchen, I think the number one problem with working with sugar is that it crystallizes, especially when you don't want it to, like when you are making quick caramel sauces, buttery salted caramel sauce, or soft and chewy sea salt caramels. Fortunately, there are tricks that you can do so that your caramels and sauces don't turn gritty.

What is sugar?
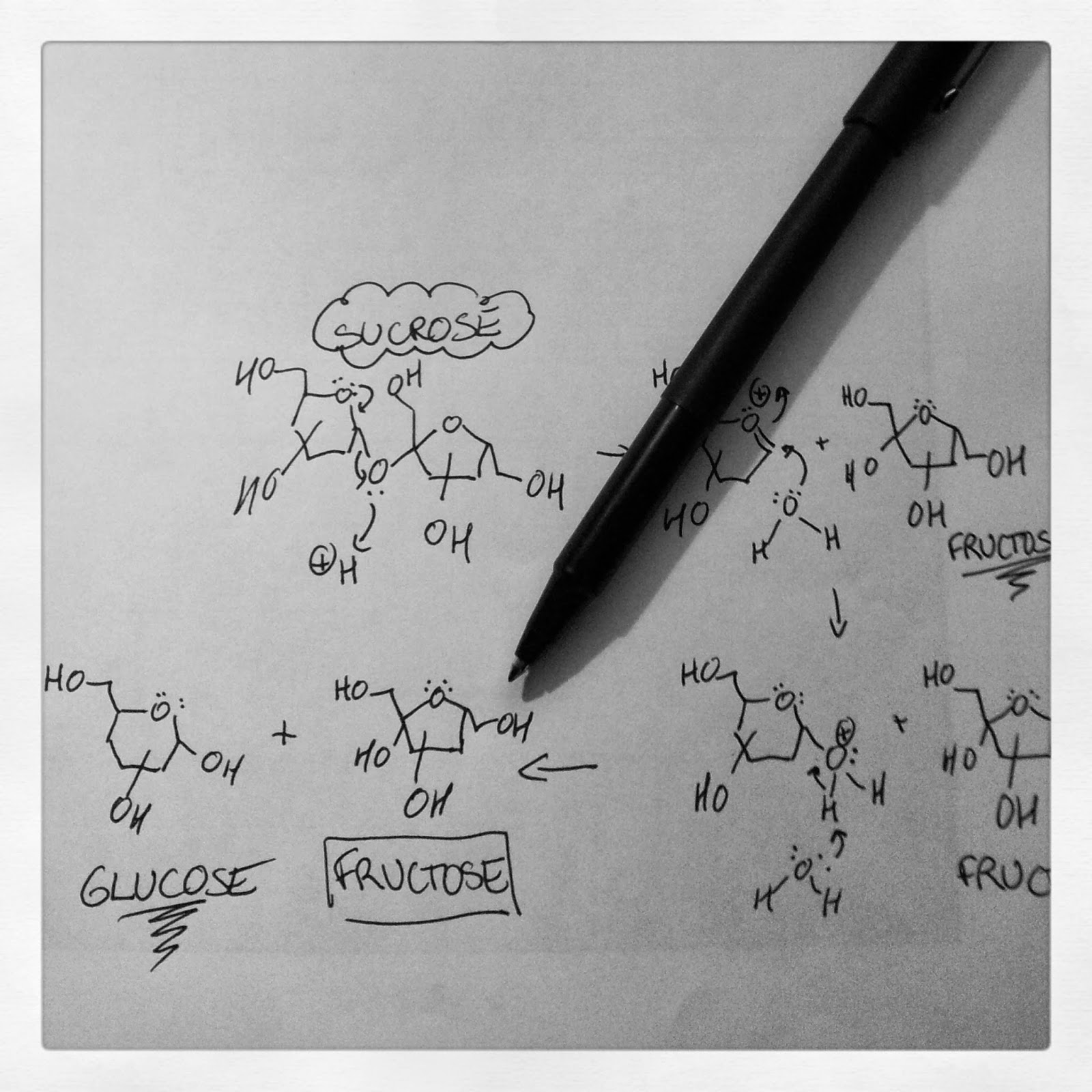
Granulated sugar is sucrose
In baking, the most common form of sugar we use in the kitchen is granulated sugar. People often assume that granulated sugar is glucose, but it's not. Glucose, along with fructose, are actually the building blocks that make up each molecule of sucrose that is granulated sugar. Brown sugar, like white sugar, is also mostly sucrose. It's important to note that in sucrose, glucose and fructose are chemically bonded.

Invert sugars such as honey, corn syrup, and glucose
The other type of sugar you need to be aware of is "invert sugar", such as corn syrup. Invert sugars are made when larger sugars, like sucrose, are broken down to their basic building blocks, glucose and fructose. What most people don't realize is that corn syrup actually comes from corn starch. Starch is the storage form of glucose in plants, and it's a long chain of glucose molecules bonded together. If you treat starch with either an enzyme (amylase) or an acid and a little heat, the starch chains break down into their building blocks: you obtain lots of glucose. Corn syrup that is readily available at grocery stores is a glucose syrup. Some corn syrups contain maltose (coming from the break-down of glucose-containing amylose starches). Honey is also an invert sugar: bees drink flower nectar containing sucrose, and they secrete an enzyme (invertase) that breaks down the sucrose (digestive acids also help this process) to form glucose and fructose. In honey, glucose and fructose are present in equal parts. You'll notice honey in these florentine cookies. Not only does it add flavour, it also ensures the sugar doesn't crystallize, leading to a gritty texture!
Corn syrup vs high-fructose corn syrup (HFCS)
Corn syrups we use at home are not to be confused with high-fructose corn syrup, which are made by treating regular corn syrup with an isomerase that converts glucose to fructose. The corn syrup you buy in grocery stores is not high fructose corn syrup.
Why do we need to know about granulated sugar and sucrose?
When sucrose is present in high concentrations, like when you are making butterscotch that's loaded with brown sugar, the sucrose molecules tend to pile up and crystallize. The molecules just can't help but crystallize because there is so much sucrose around. The caramels and quick caramels can become powdery, or even gritty (if larger crystals form) because the sucrose is essentially precipitating/crystallizing out of the sauce.

How do you stop caramel from crystallizing?
There are 2 important methods to ensure your caramel sauce doesn't crystallize when you don't want it to:
- Add an invert sugar like corn syrup or honey: The most common precaution to prevent crystallization in recipes for caramel sauces is to add an invert sugar to your recipe, like corn syrup or honey. Why? Remember invert sugars contain glucose and fructose. Sucrose has a harder time crystallizing when glucose and fructose are floating around in the saucepan because glucose and fructose prevent the sucrose molecules from piling up on each other and crystallizing. Invert sugars interfere with the crystallization of sucrose, and therefore sugar sauces and caramels are less likely to crystallize if you add a little bit of corn syrup or honey to your recipe. This is one instance when you cannot use maple syrup! Though maple syrup is often a good substitute for honey in baking, maple syrup is mostly sucrose, and therefore does not qualify as an invert sugar, nor will it help you prevent caramel sauces from crystallizing. Stick with corn syrup or honey. Glucose syrup is also a great option (you can buy it on Amazon or at specialty baking stores).
- Add an acid: If you are out of corn syrup and don't have honey on hand, you have a second option: add a squeeze of lemon juice. Lemon juice is acidic and therefore if you mix a little lemon juice with sucrose, and you heat the mixture, some of the sucrose will break down to its building blocks, i.e. glucose and fructose. By adding a little lemon juice to your sugar sauces and caramels, you are basically making a little invert sugar in your saucepan so that the sucrose, and your caramel, won't crystallize.

There are also 2 additional points to consider to avoid crystallization of caramel sauces:
- Make sure the sugar is dissolved properly before you increase the heat: when making salted caramels and caramel sauce recipes that involve caramelizing granulated sugar before adding cream and butter, I find it's very important to add a little water to your saucepan to help dissolve all the sugar crystals on low heat before heating the mixture at a higher temperature to caramelize the sugars. I find starting with water helps prevent a lot of problems later on.
- Change the order of ingredients: for caramel sauce recipes, I make sure to put the liquid in the saucepan first. Then I add the sugar on top of the liquid. I find by respecting this order, the sugar dissolves more easily and more evenly at low heat, with minimal stirring.

Methods to prevent caramel crystallization
I hope I have helped clarify the theory behind why some batches of caramel crystallize. Adding a little corn syrup to a batch of caramel makes sense on paper, but it's always nice to test out the options ourselves, so that's what I did.

It's hard to tell from the photos but the quick caramel prepared with no additives and a small amount of water is more opaque and less clear than all the others: this sauce has a powdery mouthfeel, reminiscent of brown sugar fudge. On the other hand, the caramel sauces made with either corn syrup or lemon juice are much clearer. The mouthfeel of both sauces is completely smooth, without any detectable powdery texture. Of course, the flavour of the sauce containing lemon juice was a little more citrusy, which personally I wasn't a fan of. So, lemon juice works to prevent sugar sauces from crystallizing, but perhaps the flavour might not be what you are looking for.
I tested out one more option that's not "in the books": dilution. From a practical standpoint, caramel sauces tend to crystallize because there's so much sucrose dissolved in so little water, so I doubled the amount of water to see what would happen if I made a more dilute quick caramel. Obviously the "diluted" quick caramel was a little waterier, especially next to the other batches, but from a crystallization standpoint, this sauce didn't become powdery or gritty. Of course, this option won't be of much help if you are making a real caramel because most of the water evaporates as the sugar boils, but for a quick caramel sauce, it's definitely another option worth considering. In fact, my grandmother's quick caramel sauce recipe contains double the water than the one I served with the baked apples.

Crystallization is a science
I find it ironic because as a chemist, I spent a lot of time trying to force my products to crystallize, and yet in the kitchen, we usually strive for the opposite in a perfectly smooth caramel sauce. Luckily, we have a few tricks to choose from so that we never have to face a batch of caramel turned to a solid mass of gritty sugar.
In some recipes, crystallization is a good thing!
When you are following a maple fudge recipe or making a batch of homemade maple butter, you actually want sugar crystallization to occur. These recipes use temperature to control crystallization and to make sure you form fine sugar crystals. The size of the sugar crystals can't be too large or you will end up with gritty fudge or hard maple candy.






Kecia says
How much corn syrup should I add to an existing caramel sauce recipe? If, say, the yield is 1 cup, would 1T be sufficient?
Janice Lawandi says
Great question! My starting point would be 1 tablespoon per cup (200 grams) of sugar.
I've seen less and I've seen more, but I think this is a good starting point. Hope that helps and let me know how it goes!
Katie n says
is there any way to fix the crystallization if it happens?
Janice Lawandi says
Hi Katie,
I think your best option would be to heat the mixture up again to melt the crystals. I've even seen this done for fudge that crystallizes hard and is too gritty. You can break it up and throw it back in a pot with a little extra cream and then heat it to melt and then go through the temperature points (heating to a certain temp, then cooling to another before whipping).
Rachad says
Very informative, thank you.
Can glucose syrup be substituted with powdered glucose?
What in your view the ideal proportion of glucose vs sugar?
Julie says
Hi Janice,
I am very excited about your website. I am making soft caramel candies and was told by someone that evaporated milk over cream will prevent them from crystallizing. Have you experimented with evaporated milk.
Thank you,
Lou says
This was so informative!
Thank you so much.
I really enjoyed reading it and will definitely be checking out your other articles.
I should be reading about chocolate (we are currently getting white streaks on what otherwise seems to be tempered chocolate) but I'm also just getting hooked on candy/sugar science!
Thanks again!
Mark says
Hi Janice,
I’ve had successful attempts to make caramel sauce without crystallization and the gritty mouth feel (per some of your suggestions above), but no matter what I try I can’t get it to stay soft once it gets cold - i.e once it’s drizzled over ice cream it becomes more like a chewy caramel candy which makes it difficult to eat. I’ve checked candy temperature charts and am hitting the above 350 degree mark.
Please help!
Thanks in advance.
Oppers says
So interesting, thanks for sharing.
When I am making ice cream I love them with swirled with fruits or sauces but find they crystallise, but I really like the distinction of the two flavours you get that way. So after having churned the ice cream I'll be adding in, let's say a chocolate sauce, to get that effect and pop the mix in the freezer. Is any of the above also applicable when freezing or is that another science altogether? 🙂
Janice says
Hi! That's a great question! So with ice cream making the worry is crystallization of water, i.e. ice formation, less than crystallization of sugar. If you want to add a fruit swirl to ice cream, make sure that it has enough sugar and not too much water so that the ice crystals are finer. This is where a jam or a fruit preserve would be a good choice because they are higher in sugar and have less water. If the fruit swirl is too watery, you will end up with big shards of ice in the swirl, ruining the texture. I've actually written a post about ice cream making and the ice crystals that form. It's long, but you'll find some key points: https://bakeschool.com/how-to-make-the-best-ice-cream/
Hope that helps!
Shawn says
Here's a weird question... Could you add invertase to cooled caramel or butterscotch to prevent (or reverse) crystallization?